Giving cells something to cling to with Hydrogel microdroplets
Encapsulating biological material in hydrogel droplets is a precise and complex task, requiring the latest approaches in both cell culturing and microfluidic technology. So why is this technique considered to be the present and future of many cellular biology applications?
2D or not 2D, that is the biological question
In classical development, one would be hard pressed to find many cell types which do not grow in the supporting meshwork of some kind of extracellular matrix, be that the familiar ECM found in multicellular organisms or the supportive biofilms secreted by prokaryotes. Very few cell types grow and develop in vivo on a two-dimensional solid surface, meaning that the environment provided by classical well-plate cell culture methods is generally a poor substitute for in vivo studies. Systems such as hanging drop cultures go a long way towards creating more naturalistic and three-dimensional cell growth environments (1). Unfortunately, due to their suspended nature, they are finicky to produce and maintain, and once formed do not easily allow the further perfusion of molecules, for example to introduce nutrients, gases or drug molecules.
What are hydrogels and what are they used for?
Hydrogels are generally understood as crosslinked scaffolds of long protein strands which freely allow the movement of aqueous solutions through their structures. The majority of a hydrogel’s volume is made up by empty space, whilst still giving ample support to encased cells. Two particular hydrogels are of special interest to the field of single cell microdroplet encapsulation, agarose and Matrigel. These take centre-stage in biological pursuits due to their inherent biocompatibility. Unlike synthetic polymer scaffolds, cells grow more readily in these hydrogels which, like cells themselves, are biological in origin (2).
Staying single: keeping cell’s identity within hydrogels
Suspending cells in three-dimensional hydrogels provides cells with both a supporting scaffold and an option to have nutrients and drug molecules perfused through to them. The mass seeding of single cells into a continuous block of hydrogel is a tempting methodology for tissue engineering or the modeling of organs. However, both clinical and basic research is increasingly focused on the growth and interactions of single cells, for example to understand the complex heterogeneity within a tumor. This requires the separation of cells to maintain monoclonal identity.
With all this in mind, the holy grail of cell culture methods would combine the high throughput encapsulation of cells in a 3D scaffold with the free perfusion of molecules whilst also compartmentalizing the cells to maintain their monoclonal identity.
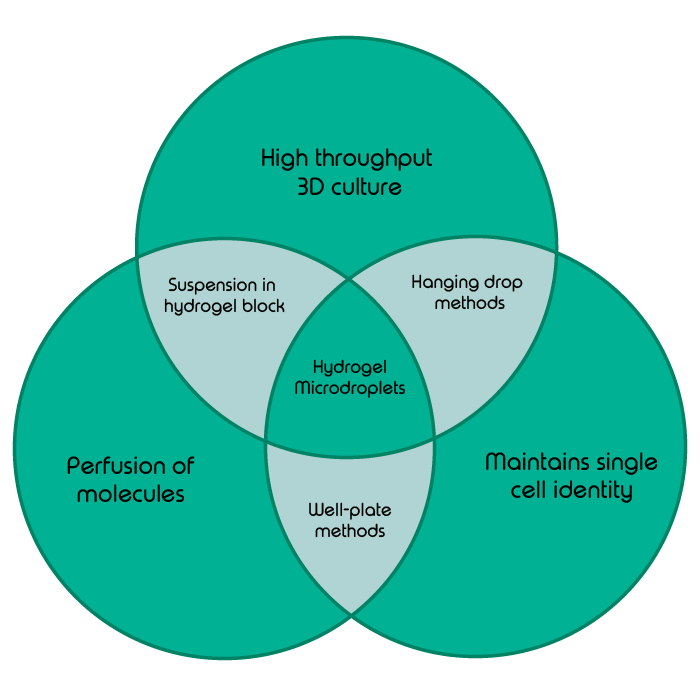
Don’t agonize, dropletize – the benefits of encapsulating cells in hydrogel droplets
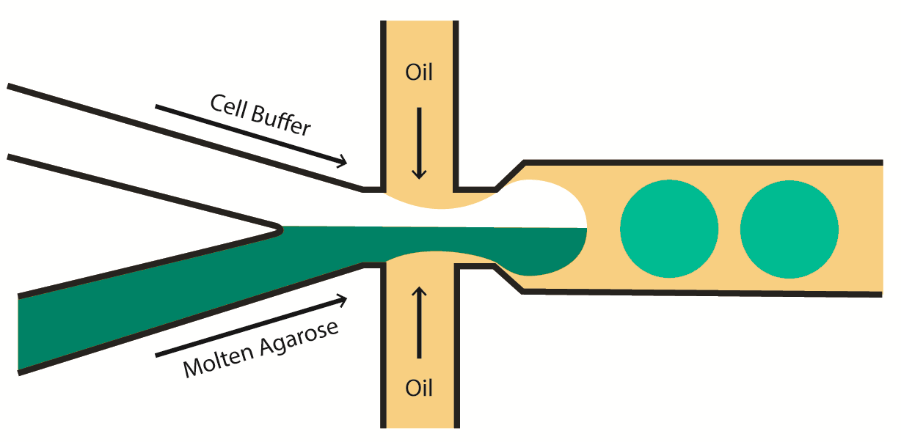
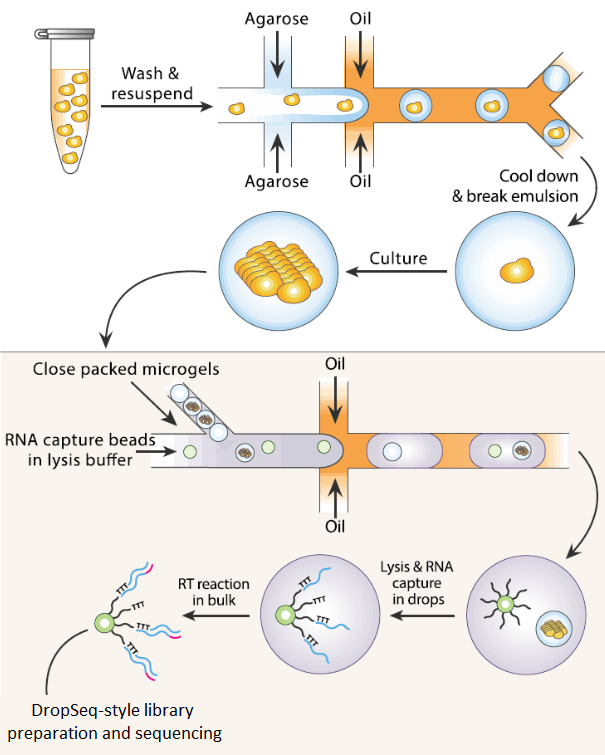
Cells can be continuously captured in droplets containing hydrogel using droplet microfluidics, which surrounds tiny pockets of molten agarose containing cells in cell buffer with an immiscible oil shell. The hydrogel droplets, once hardened and removed from their oil shells, are spherical scaffolds which remain stable in an aqueous solution such as cell buffer. Cells suspended in these droplets benefit both from the free perfusion of nutrients and the support provided by a three-dimensional meshwork. Immobilised, these cells can also be later assayed on a single cell level without the need for manual separation (3).
A workflow is only as good as its tools – so how can you implement this technique?
So, what is crucial in terms of technology and instrumentation for us to take advantage of cellular encapsulation within hydrogel microdroplets?
In order to make this technique a valid step up from well plate-based methods, droplet-based hydrogel capture must achieve high-throughput via automation. Generating these droplets in a stable microfluidic manner introduces an astounding level of scalability to this cellular technique, the only factor limiting cell number being time and reagent availability.
Precise and wide-ranging temperature control is a necessity, to ensure that the solid-liquid phase change which occurs during hydrogel gelation is strictly controlled. Also, droplets generated in this manner must display a high level of monodispersity (ie. low variation in the diameter of each droplet) to ensure that each cell is cultured in the same volume of hydrogel. This allows the predictable allocation of cells into each droplet to best avoid cell doublets.
Generating hydrogel microdroplets continuously using microfluidic droplet-based culture methods often enables cost savings. Growing cells in droplets requires less media and reagents overall than equivalent microwell methods (2). It similarly saves time by avoiding the laborious process of growing a single cell into a colony big enough to visualize and pick (a chore familiar to most microbiologists).
Intrigued? Find out how all this is possible with our Nadia Innovate
Beyond single cell culture – what other applications can hydrogels help achieve?
Whilst its merit as a single cell culture methodology is extremely promising, hydrogel microdroplet encapsulation is compatible with a slew of wide-ranging potential applications (4).
The ability of microfluidic devices to incorporate more than one cell type into droplets allows further interesting developments. Firstly, cell co-encapsulation of two distinct cell types can create precise niches for some cell types to develop in. This is specifically necessary if the growth of one cell type is dependent upon an auxiliary or helper cell type. Such setups could feasibly be used to study cell-cell signalling or provoke cell fates in previously unattainable throughput and scale (5).
Secondly, hydrogel droplets can be sorted along with their contents using flow cytometry (6). By co-encapsulating two distinct cell types, with one cell type acting as fluorescent reporter for a secreted substance from a cell type of interest, FACS can be performed not only on cells, but also their local microenvironments. This, coupled with the capacity to easily culture and image singulated cells, means that hydrogel encapsulation holds great potential for bacterial isolation and analysis (7).
Remarkably, the precision of modern microfluidic system allows objects even smaller than cells to be partitioned into single droplets. Even single molecules, such as oligonucleotide fragments or drug components, can be entrapped alongside single cells, standing to revolutionise future molecular techniques (3).
Keeping in step with the times
The ability of agarose and other hydrogels to immobilize nucleic acids and other molecules can allow step changes and multiple consecutive reactions to be induced in single droplets without having to include all reagents in the initial encapsulation. Conceptually, this may not seem like such a vast step forward from simply performing each of these stepwise reactions sequentially in a classic microcentrifuge tube. However, the formation of solid individual microdroplets means that single cellular identity can be maintained throughout the entire process whilst still retaining high-throughput (8). This potential for combinatorial workflows culminates in applications such as Isogenic Colony Sequencing (ICO-Seq). In such hydrogel droplet applications, single cells can be cultured over time and then reintroduced to DropSeq-style sequencing workflows in a high throughput process which maintains each colony’s distinct single cellular origin (9).
Pharmaceutical development through screening of organoids
The pharmaceutical development of therapeutic molecules is particularly set to remain dependent on large-scale screens on cultured cells. The screening of cells confined to microdroplets by hydrogel microfluidics allows throughput limited only by cell number, whilst also maintaining rigorous cellular compartmentalisation (4). Whilst expensive, Matrigel™ is generally held as the gold-standard for these applications, as it is most apt for mimicking in vivo cell structures. Biologically-interesting cell agglomerations originating from a single seeding cell, such as organoids or spheroids, can be generated and screened en masse using this technique (10).
Going green- the possibilities for plant research
On the more floral side of biology, the study of plant cells often focuses on their cellular interactions with either abiotic molecules (such as pesticides) or pathogens (such as viruses or bacteria). Being able to co-encapsulate all these different cell-like objects in predictable ratios via microfluidic flow focusing allows vast tracts of plant and crop research to branch into single cell analyses.
Additionally, plant cells often derive their cellular identity from their position in relation to their neighbors. They, therefore, have a propensity to derive cellular responses from perturbations of mechanical attachments. In plant cell culture, this means that limiting potential surface attachments is paramount for reproducible single cell plant studies (11). Microfluidic droplet-based hydrogel encapsulation techniques do just this, by separating singulated protoplasts not only from their fellow cells, but also from solid potentially adherent surfaces. This is further aided by microfluidic flow systems reproducibly creating perfectly spherical droplets, devoid of irregularities which may produce different mechanical strain patterns in different droplets.
In a truly historic field like plant culturing, mired in the tradition of older manual techniques, adoption of novel technologies is limited. Therefore, many areas of plant research stand to advance rapidly by joining the single cell revolution bolstered by techniques such as hydrogel microdroplets.
Learn more about plant protoplast RNA-Seq with the Nadia platform
Encapsulation in hydrogel microdroplets: a budding technology
Hydrogel microdroplets are yet to reach their zenith in terms of their utility for many subfields within single cell biology. The ability to generate discrete biological culture vessels at such a blistering rate is in many ways leaps and bounds ahead of many fields yet to embrace this exciting technique.
If you’d like to find out more about the generation of hydrogel droplets using microfluidics, refer to our Application Note Agarose Encapsulation.
References
- Foty R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J Vis Exp. 2011;(51).
- Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev [Internet]. 2008 Mar [cited 2019 Oct 8];14(1):61–86. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18454635
- Zhu Z, Yang CJ. Hydrogel droplet microfluidics for high-throughput single molecule/cell analysis. Acc Chem Res. 2017;50(1):22–31.
- Luo R-C, Chen C-H. Structured Microgels through Microfluidic Assembly and Their Biomedical Applications. Soft. 2012;01(01):1–23.
- Tumarkin E, Tzadu L, Csaszar E, Seo M, Zhang H, Lee A, et al. High-throughput combinatorial cell co-culture using microfluidics. Integr Biol (Camb) [Internet]. 2011 Jun [cited 2019 Oct 8];3(6):653–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21526262
- Bai Y, Weibull E, Joensson HN, Andersson-Svahn H. Interfacing picoliter droplet microfluidics with addressable microliter compartments using fluorescence activated cell sorting. Sensors Actuators, B Chem. 2014 Apr;194:249–54.
- Eun Y-J, Utada AS, Copeland MF, Takeuchi S, Weibel DB. Encapsulating Bacteria in Agarose Microparticles Using Microfluidics for High-Throughput Cell Analysis and Isolation. ACS Chem Biol [Internet]. 2011 Mar 18;6(3):260–6. Available from: https://doi.org/10.1021/cb100336p
- Zhang H, Jenkins G, Zou Y, Zhu Z, Yang CJ. Massively parallel single-molecule and single-cell emulsion reverse transcription polymerase chain reaction using agarose droplet microfluidics. Anal Chem. 2012 Apr 17;84(8):3599–606.
- Liu L, Dalal CK, Heineike BM, Abate AR. High throughput gene expression profiling of yeast colonies with microgel-culture Drop-seq. Lab Chip. 2019;19(10):1838–49.
- Li Y, Kumacheva E. Hydrogel microenvironments for cancer spheroid growth and drug screening. Sci Adv [Internet]. 2018 [cited 2019 Oct 8];4(4):eaas8998. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29719868
- Grasso MS, Lintilhac PM. Microbead encapsulation of living plant protoplasts: A new tool for the handling of single plant cells. Appl Plant Sci [Internet]. 2016 May [cited 2019 Oct 8];4(5). Available from: http://www.ncbi.nlm.nih.gov/pubmed/27213126